CHM 130 Introduction to Chemistry at Arizona College of Nursing
Access The Exact Questions for CHM 130 Introduction to Chemistry at Arizona College of Nursing
💯 100% Pass Rate guaranteed
🗓️ Unlock for 1 Month
Rated 4.8/5 from over 1000+ reviews
- Unlimited Exact Practice Test Questions
- Trusted By 200 Million Students and Professors
What’s Included:
- Unlock Actual Exam Questions and Answers for CHM 130 Introduction to Chemistry at Arizona College of Nursing on monthly basis
- Well-structured questions covering all topics, accompanied by organized images.
- Learn from mistakes with detailed answer explanations.
- Easy To understand explanations for all students.

Study smarter, pass faster! Get 30-day access to comprehensive CHM 130 Introduction to Chemistry WGU exam questions. Our materials ensure your success.
Free CHM 130 Introduction to Chemistry at Arizona College of Nursing Questions
An active site that makes a conformation change to accommodate a substrate is associated with which enzyme action mechanism?
-
Allosteric Theory
-
Zymogen Theory
-
Lock-and-Key Theory
-
Induced Fit Theory
Explanation
Correct Answer: D. Induced Fit Theory
Explanation of Correct Answer:
The Induced Fit Theory proposes that when a substrate binds to an enzyme, the enzyme’s active site changes shape slightly to better accommodate the substrate. This dynamic adjustment enhances binding precision and catalytic efficiency. In contrast, the Lock-and-Key Theory suggests a rigid fit between enzyme and substrate, while Allosteric Theory involves regulation at a site other than the active site, and Zymogen Theory pertains to inactive enzyme precursors that require activation.
What is the name of the following organic compound: CH₃CH=CHCH₂CH₃
-
2-hexene
-
2-pentene
-
3-pentene
-
2-pentadiene
Explanation
Correct Answer: B. 2-pentene
Explanation of Correct Answer:
The structure CH₃CH=CHCH₂CH₃ is a five-carbon chain with one double bond, so the parent name is pentene. Number the chain from the end closest to the double bond. The double bond starts between carbons 2 and 3, so the correct IUPAC name is 2-pentene. 3-pentene is not used because numbering must give the lowest possible locant for the double bond. Choices with six carbons or multiple double bonds are not correct.
Identify the functional group.
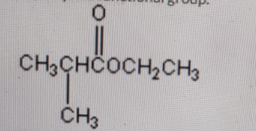
-
Ketone
-
Carboxylic acid
-
Ester
-
Aldehyde.
Explanation
Correct Answer: A. Ketone
Explanation of Correct Answer:
The structure shows a carbonyl group (C=O) bonded to two carbon atoms — one from the left (CH₃CH–) and one from the right (CH₂CH₃). This arrangement defines a ketone functional group. In contrast, aldehydes have the carbonyl carbon attached to at least one hydrogen, carboxylic acids include a –COOH group, and esters have a –COOR linkage. Therefore, this compound clearly contains a ketone group.
Determine the IUPAC name of this structure:
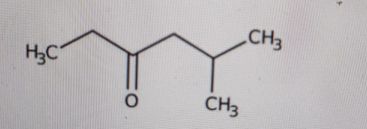
-
3-pentanone-5-methyl
-
5-methyl-3-hexanone
-
2-methyl-4-hexanone
-
5-methyl-pentanal
Explanation
Correct Answer: B. 5-methyl-3-hexanone
Explanation of Correct Answer:
First, identify the longest continuous carbon chain that includes the carbonyl carbon. The chain has six carbons, so the parent name is hexanone. The carbonyl (C=O) is in the middle of the chain, on carbon 3, so that gives us 3-hexanone. Next, look at the right side of the chain: that carbon has an extra CH₃ branch. That branch is on carbon 5 of the main chain. A methyl group on carbon 5 gives 5-methyl. Put it all together: 5-methyl-3-hexanone.
Name the following functional group presented in this compound.
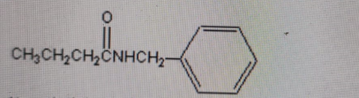
-
Amine
-
Amide
-
Alcohol
-
Ester
Explanation
Correct Answer: B. Amide
Explanation of Correct Answer:
The structure shows a carbonyl group (C=O) directly attached to a nitrogen (–C(=O)–NH–). This is the defining feature of an amide functional group. An amine would have a nitrogen attached to carbon without a carbonyl. An alcohol would contain an –OH group, which is not present here. An ester would have –C(=O)–O–R, with an oxygen bonded to the carbonyl carbon, which this compound does not have.
Which of the following is a disaccharide?
-
Glucose
-
Galactose
-
Fructose
-
Maltose
Explanation
Correct Answer: D. Maltose
Explanation of Correct Answer:
Maltose is a disaccharide composed of two glucose molecules joined by a glycosidic bond. Disaccharides form when two monosaccharides undergo a dehydration (condensation) reaction, releasing water. Maltose is commonly produced during the breakdown of starch in digestion or fermentation. In contrast, glucose, galactose, and fructose are single-unit sugars (monosaccharides) that cannot be hydrolyzed into simpler sugars.
What is the name of the following compound: HOCH₂CH₂CH₂CH₂OH
-
1,4-butanediol
-
1,4-butanol
-
1,3-butanol
-
1,1-butanediol
Explanation
Correct Answer: A. 1,4-butanediol
Explanation of Correct Answer:
The compound HOCH₂CH₂CH₂CH₂OH has four carbon atoms (butane backbone) and two hydroxyl (–OH) groups located on the first and fourth carbons. The “diol” suffix indicates the presence of two hydroxyl groups, and the numbers specify their positions. Therefore, its correct IUPAC name is 1,4-butanediol. The other names are incorrect because they either misplace or reduce the number of hydroxyl groups present in the molecule.
What are the three parts of an amino acid?
-
Phosphate group, amino group, and organic R group
-
Phosphate group, sugar, and organic R group
-
Carboxyl group, amino group, and organic R group
-
Phosphate group, amino group, and carboxyl group
Explanation
Correct Answer: C. Carboxyl group, amino group, and organic R group
Explanation of Correct Answer:
An amino acid contains three essential components: an amino group (–NH₂), a carboxyl group (–COOH), and an organic R group (side chain). The amino group acts as a base, the carboxyl group acts as an acid, and the R group determines the specific chemical properties of each amino acid. Together, these groups allow amino acids to link via peptide bonds to form proteins.
What is the correct structure of N,N-dimethylpropanamide?
-
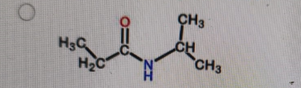
-
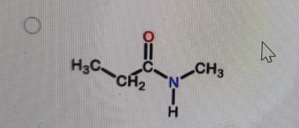
-
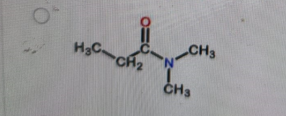
-
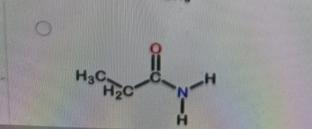
Explanation
Correct Answer: B. (second structure) — CH₃CH₂–C(=O)–N(CH₃)₂
Explanation of Correct Answer:
N,N-dimethylpropanamide is an amide derived from propanoic acid (propan-) with the –CONH₂ group converted so that both hydrogens on the nitrogen are replaced by methyl groups. That gives –C(=O)–N(CH₃)₂. The carbonyl is attached to CH₃CH₂– (the propan- part). Option B is the only structure showing an amide with two methyl groups bonded to the nitrogen and no hydrogens on that nitrogen.
Comparing the solubilities of the two compounds 1-hexanamine and heptane. 1-hexanamine is ______
-
not soluble in water, while heptane is
-
less soluble in water than heptane
-
equally soluble in water as heptane
-
more soluble in water than heptane
Explanation
Correct Answer: D. more soluble in water than heptane
Explanation of Correct Answer:
1-hexanamine is more soluble in water than heptane because it contains an –NH₂ (amine) group capable of forming hydrogen bonds with water molecules. This polar interaction enhances its solubility. Heptane, on the other hand, is a nonpolar hydrocarbon lacking any functional groups that can interact with water, making it highly hydrophobic and virtually insoluble. Therefore, the polar nature of 1-hexanamine explains its greater solubility in water.
How to Order
Select Your Exam
Click on your desired exam to open its dedicated page with resources like practice questions, flashcards, and study guides.Choose what to focus on, Your selected exam is saved for quick access Once you log in.
Subscribe
Hit the Subscribe button on the platform. With your subscription, you will enjoy unlimited access to all practice questions and resources for a full 1-month period. After the month has elapsed, you can choose to resubscribe to continue benefiting from our comprehensive exam preparation tools and resources.
Pay and unlock the practice Questions
Once your payment is processed, you’ll immediately unlock access to all practice questions tailored to your selected exam for 1 month .